What Does The Autoimmune Brain Panel™ Measure?
Autoantibody testing for neuropsychiatric disorders
The Autoimmune Brain Panel™ (also known as the Cunningham Panel™) is a series of five proprietary, validated tests. Four of these tests measure circulating levels of specific autoantibodies in a patient’s serum at the time the specimen is drawn.
The fifth test, the CaMKII assay, measures the ability of a patient’s autoantibodies to stimulate this enzyme, resulting in an upregulation (or increase) of brain neurotransmitters such as dopamine, epinephrine and norepinephrine. This increase can trigger a variety of neurologic and/or psychiatric symptoms.
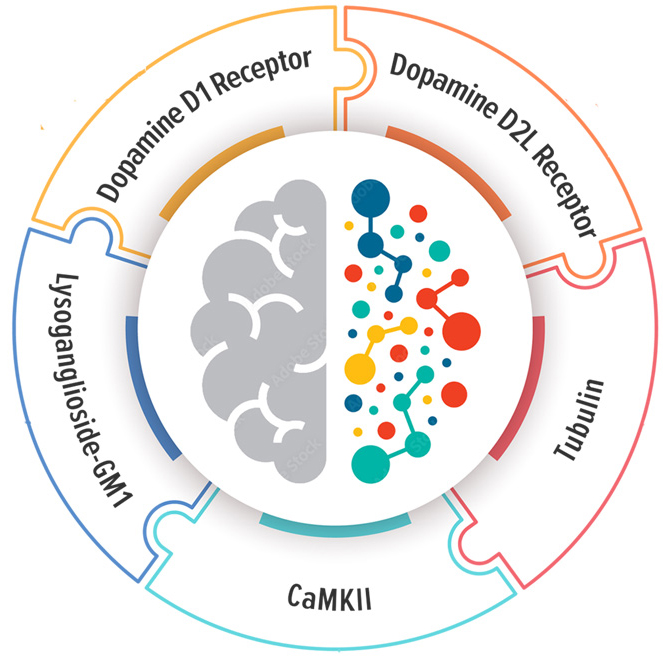
Autoimmune Brain Panel™ Includes 5 Individual Tests
The neuronal targets included in the Autoimmune Brain Panel™ were selected based upon their biological association with specific neurologic and psychiatric symptoms.
Background on Dopamine Receptors
The dopamine receptors (D1, D2, D3, D4, D5) are widely distributed in the brain and mediate the effects of dopamine on: cognition, emotion, regulation of hunger, satiety, locomotor activity and on the endocrine system. 1 Dopamine receptors are highly concentrated on synaptic neurons in the brain.
The dysregulation of the dopaminergic system has been linked to the pathophysiology of many diseases, such as schizophrenia, attention deficit/hyperactivity disorder, and depression. 1
Autoantibodies against Dopamine D1 Receptor
When a patient’s autoantibodies are directed against this receptor, they can interfere with the normal function of this receptor by stimulating the receptor or by blocking the ability of dopamine to bind to this receptor.
This disruption of dopamine transmission can lead to the manifestation of various neuropsychiatric disorders. 2, 3
Individuals with elevated levels of anti-Dopamine D1 antibodies often reported having psychiatric symptoms including psychosis, OCD behaviors and tics, based upon our clinical laboratory patient population analysis.
Background on Dopamine Receptors
The dopamine receptors (D1, D2, D3, D4, D5) are widely distributed in the brain and mediate the effects of dopamine on: cognition, emotion, regulation of hunger, satiety, locomotor activity and on the endocrine system. 1 Dopamine receptors are highly concentrated on synaptic neurons in the brain.
The dysregulation of the dopaminergic system has been linked to the pathophysiology of many diseases, such as Alzheimer’s disease, schizophrenia, Parkinson’s disease, attention deficit/hyperactivity disorder, depression and drug addiction. 1
Autoantibodies against Dopamine D2 Receptor
Autoantibodies directed against this receptor can interfere with its normal function through overstimulation or by blocking dopamine’s normal binding with the receptor.
Autoantibodies directed against D2 receptors correlated with various neuropsychiatric symptoms. 4
Individuals with elevated levels of anti-Dopamine D2 antibodies often reported having symptoms involving uncontrolled motor movements, such as hyperactivity and impulsivity, based on analysis of our clinical laboratory population.
Autoantibodies Against Lysoganglioside-GM1
Lysoganglioside GM1 is located within the membrane of nerve cells and is highly concentrated in the central nervous system. It functions as insulation around the nerve cell and plays an important role in signal transmission in the brain. It provides a vital function in communication between neurons and ensures proper transmission of impulses.
When a patient’s autoantibodies are directed against lysoganglioside, they can interrupt this communication and interfere with normal nerve transmission.
Individuals with elevated levels of anti-Lysoganglioside GM1 often reported having joint pain, connective tissue pain, headaches, and other types of neuropathy, based upon our clinical laboratory patient population analysis.
An autoimmune response against ganglioside GM1 has been implicated in Guillain-Barré Syndrome. 4,5,6
Autoantibodies Against Tubulin
Tubulin is an intracellular protein that forms microtubules providing a skeleton for maintaining cell shape and is thought to be involved in cell motility and intracellular transport.
Tubulin is contained within most every cell and is highly abundant and concentrated in brain cells. It plays an important role in cell signaling and communication within the cell.
When a patient’s autoantibodies are directed against Tubulin, OCD-like symptoms and cognitive impairment, such as ‘brain fog’ have been reported, based upon our clinical laboratory patient population analysis.
CaMKII – a Cell Stimulation Assay
Calcium/calmodulin-dependent protein kinase II (CaMKII) is a key enzyme that is involved in the upregulation of neurotransmitters: dopamine, epinephrine and norepinephrine.
CaMKII is involved in regulating various neuronal functions, such as neurotransmitter synthesis and release, receptor signaling, and long-term synaptic plasticity. It plays a crucial role in synaptic transmission and transmitter release.
The CaMKII test performed in the Autoimmune Brain Panel™ is a “cell stimulation assay.” This test involves growing human brain cells in culture and incubating the patient’s serum on these cells to determine if autoantibodies that are present bind to and stimulate this enzyme.
If a patient’s autoantibodies stimulate this enzyme, it can trigger abnormal neurologic, psychiatric and behavioral symptoms. 7.
Elevated levels of CaMKII tend to indicate the presence of active infection of some type.
Interpreting test results
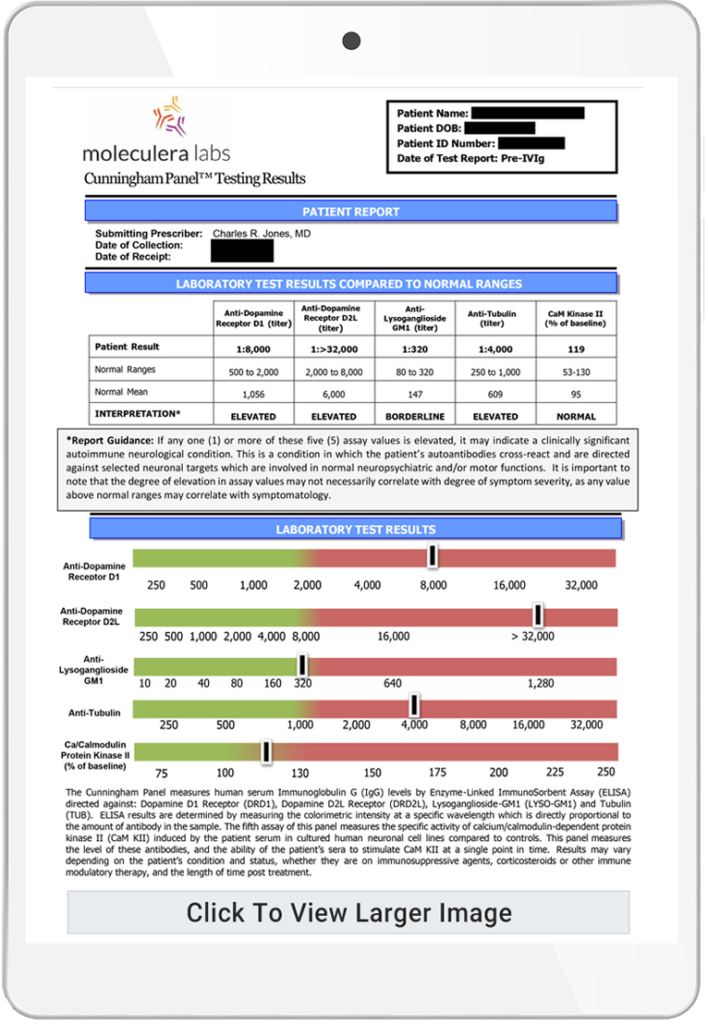
The Autoimmune Brain Panel™ is considered positive if one or more of these individual test results exceed their normal ranges. The autoantibody test results are expressed as titers (or final dilution) at which an endpoint was observed on an Enzyme-Linked Immunosorbent Assay (ELISA) format. The CaMKII is a cell stimulation assay, which measures the stimulatory ability of a patient’s autoantibody IgGs to increase the activity of the CaMKII enzyme within a human brain cell line. The result is a numeric score that reflects the percent above or below baseline activity.
Identifying autoimmune dysfunction
The Autoimmune Brain Panel™ results can aid a clinician’s diagnosis with supportive laboratory evidence of an autoimmune dysfunction directed against certain biological targets in the brain.
By identifying an underlying autoimmune dysfunction, these results can assist the clinician in selecting an appropriate treatment regimen.
References
- Chain Jennifer L., Alvarez Kathy, Mascaro-Blanco Adita, Reim Sean, Bentley Rebecca, Hommer Rebecca, Grant Paul, Leckman James F., Kawikova Ivana, Williams Kyle, Stoner Julie A., Swedo Susan E., Cunningham Madeleine W. “Autoantibody Biomarkers for Basal Ganglia Encephalitis in Sydenham Chorea and Pediatric Autoimmune Neuropsychiatric Disorder Associated With Streptococcal Infections.” Frontiers In Psychiatry, vol. 11, 2020, p. 564.
- Ben-pazi, H., J.A. Stoner, and M.W. Cunningham, Dopamine receptor autoantibodies correlate with symptoms in Sydenham’s chorea. PLoS One, 2013. 8(9): p. e73516
- Cunningham, M.W. and C.J. Cox, Autoimmunity against dopamine receptors in neuropsychiatric and movement disorders: a review of Sydenham chorea and beyond. Acta Physiol (Oxf), 2016. 216(1): p. 90-100.
- Pukin AV, Jacobs BC, Tio-Gillen AP, Gilbert M, Endtz HP, van Belkum A, Visser GM, Zuilhof H. Detection of antibodies in neuropathy patients by synthetic GM1 mimics. Glycobiology. 2011 Dec;21(12):1642-50.
- Yu RK, Usuki S, Ariga T. Ganglioside molecular mimicry and its pathological roles in Guillain-Barré syndrome and related diseases. Infect Immun. 2006;74(12):6517-6527.
- Khalili-Shirazi A, Gregson N, Gray I, Rees J, Winer J, Hughes R. Antiganglioside antibodies in Guillain-Barré syndrome after a recent cytomegalovirus infection. J Neurol Neurosurg Psychiatry. 1999 Mar;66(3):376-9.
- Kirvan, C. A., Swedo, S.E., Heuser, J.S., Cunningham, M.W. (2003). “Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea.” Nature Medicine 9(7): 914-920.