Autoimmune Brain Panel™ Sensitivity and Specificity
Accuracy of the Panel
Study findings demonstrated a strong positive association between changes in neuropsychiatric symptoms and changes in the level of anti-neuronal antibodies and antibody-mediated CaMKII human neuronal cell activation.
Based upon the change in the number of positive tests, the overall accuracy was 86%, the sensitivity and specificity were 88% and 83% respectively. The study results suggest there may be clinical utility in monitoring autoantibody levels and stimulatory activity against these five neuronal targets when diagnosing and treating patients with immune-mediated neuropsychiatric disorders.
Empowering clinicians with actionable results.
Numerous studies have linked movement, behavior, and neuropsychiatric disorders to infections and the production of anti-neuronal autoantibodies.
In a clinical study, we found that changes in autoantibody levels directed against neuronal targets in the brain and cell stimulatory activity, as measured with the Autoimmune Brain Panel™, correlated with pre- and post-treatment neuropsychiatric symptoms.
Results you can count on.

Panel predicts IVIG treatment response
A separately published clinical study evaluated the effectiveness of the Autoimmune Brain Panel™ in predicting treatment outcomes for a subset of patients with autism who also suffered from autoimmune encephalopathy.
Connery et al. found that the Autoimmune Brain Panel™ predicted a patient’s positive response to intravenous immunoglobulin (IVIG) treatment with a sensitivity of 90% to 100%, a specificity of 67% to 75%, and an overall accuracy between 81% and 88%. 2
Case study: Autism and IVIG
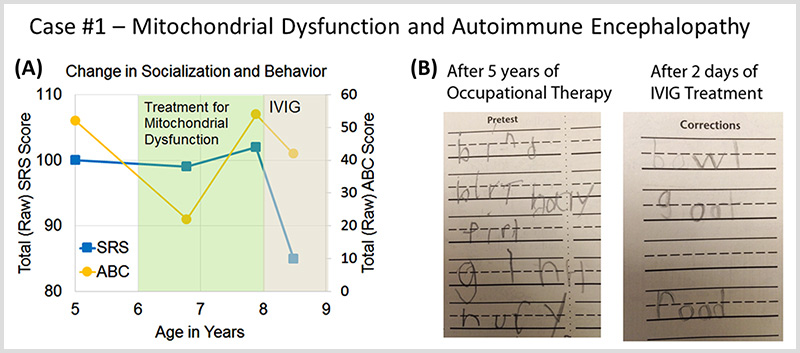
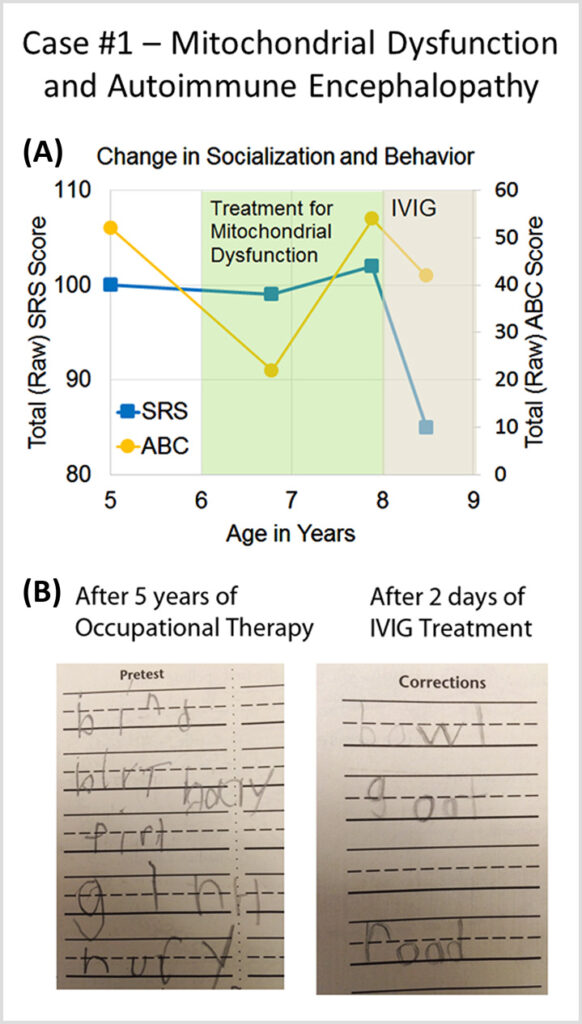
Case #1 describes a patient with autism spectrum disorder and mitochondrial dysfunction who developed a sudden worsening in behavior at age 8 and was diagnosed with autoimmune encephalopathy.
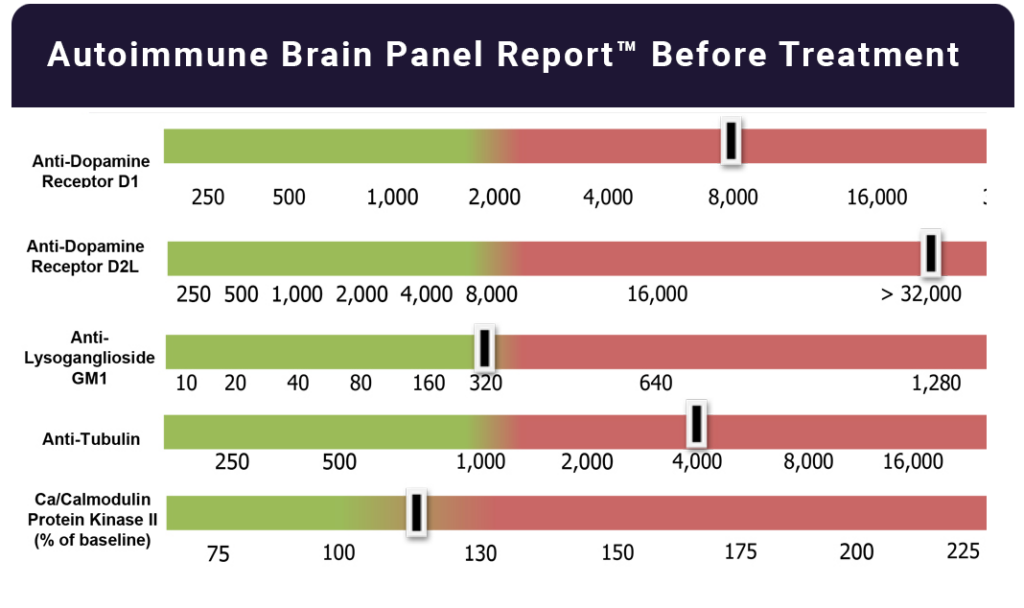
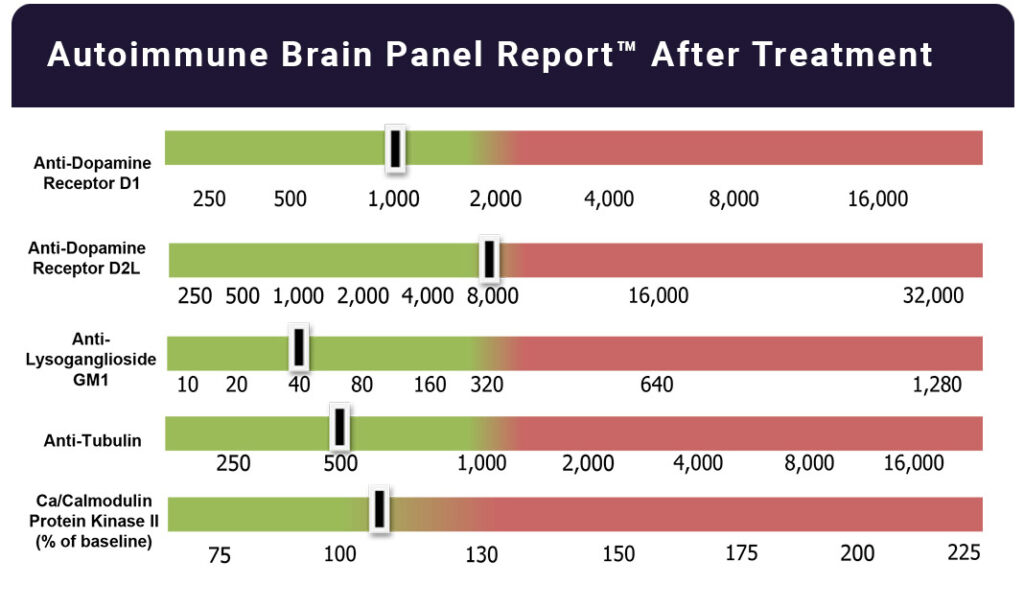
Autoimmune Brain Panel™ test results showed elevations in anti-tubulin antibodies (2000; nl = 250–1000) and CaMKII (151, nl < 130), prompting a trial of IVIG at 1 g/kg/day × 2 days every month. Within 2 days of the IVIG treatment, his handwriting significantly improved. With each subsequent treatment, his socialization and language skills improved.

Test identifies autoimmune encephalitis in children
Another study indicates antineuronal antibody biomarkers and CaMKII activation, comprising the Autoimmune Brain Panel™ (also known as the Cunningham Panel™), may be a useful adjunct to clinical diagnosis of Sydenham chorea, PANDAS and related disorders and are the first known group of autoantibodies detecting dopamine receptor-mediated encephalitis in children. 3
References
- Shimasaki C, Frye RE, Trifiletti R, Cooperstock M, Kaplan G, Melamed I, Greenberg R, Katz A, Fier E, Kem D, Traver D, Dempsey T, Latimer ME, Cross A, Dunn JP, Bentley R, Alvarez K, Reim S, Appleman J. Evaluation of the Cunningham Panel™ in pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS) and pediatric acute-onset neuropsychiatric syndrome (PANS): Changes in antineuronal antibody titers parallel changes in patient symptoms. J Neuroimmunol. 2020 Feb 15;339:577138. doi: 10.1016/j.jneuroim.2019.577138. Epub 2019 Dec 15. PMID: 31884258.
- Connery, K. et al. Intravenous immunoglobulin for the treatment of autoimmune encephalopathy in children with autism. Transl. Psychiatry 8, 148 (2018). doi: https://pubmed.ncbi.nlm.nih.gov/30097568/
- Chain Jennifer L., Alvarez Kathy, Mascaro-Blanco Adita, Reim Sean, Bentley Rebecca, Hommer Rebecca, Grant Paul, Leckman James F., Kawikova Ivana, Williams Kyle, Stoner Julie A., Swedo Susan E., Cunningham Madeleine W. “Autoantibody Biomarkers for Basal Ganglia Encephalitis in Sydenham Chorea and Pediatric Autoimmune Neuropsychiatric Disorder Associated With Streptococcal Infections.” Frontiers In Psychiatry, vol. 11, 2020, p. 564., doi: https://www.frontiersin.org/article/10.3389/fpsyt.2020.00564